IMPURE SUBSTANCES
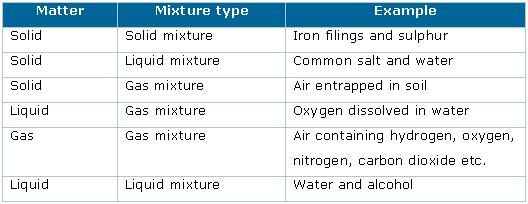
Impure substances are commonly called mixtures. A mixture is a material containing two or more elements or compounds that are in close contact and are mixed in any proportion. The constituents of mixtures exhibit their individual properties. These constituents can be separated by physical means. Example: air, gunpowder, etc.
Mixtures can be homogeneous or heterogeneous. A homogeneous mixture has a uniform composition through out its mass. For example, sugar or salt dissolved in water, alcohol in water, etc. While in a heterogeneous mixture the composition is not uniform throughout its mass. Different portions of a heterogeneous mixture show different properties. There are visible sharp boundaries. Example: Oil and water, salt and sand, etc.
We can now summarize the properties of mixtures as follows:
- A mixture may be homogenous or heterogeneous.
- The constituents of a mixture can be separated by physical means like filtration, evaporation, sublimation and magnetic separation.
- In the preparation of a mixture, energy is neither evolved nor absorbed.
- A mixture has no definite melting and boiling point.
-The constituents of a mixture retain their original set of properties. For example, magnet attracts iron filings in a mixture of sand and iron powder.
Types of Mixtures